How should we define a good disposable medical product? Is a good disposable medical product just refer to the good quality? Kohope Medical Devices Co., Ltd. would like to tell you that based on high quality, safety medical supplies also pursue a wonderful sense of use, convenient follow-up treatment, and environmental protection.
Different size, meet different requirements
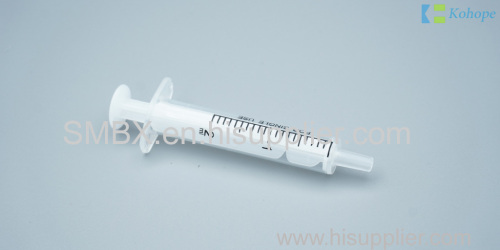
Centric or side nozzle, clear and transparent barrel
Leakage-proof plunger guarantee, special design to prevent the plunger from slipping out
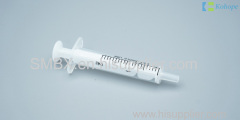
Introduction Of 2 Part Syringes
The two part syringe is assembled by barrel, plunger, with/without hypodermic needle.
There is a constriction near the barrel's handles which is a well-defined plunger stop and can effectively prevent the plunger from accidentally pulling out during use.
The hypodermic syringes used together with hypodermic needles are intended to inject the drug for the patient or take out the blood for the patient.
Specifications Of 2 Part Syringes
Details Of 2 Part Syringes
Main material: PP, SUS304 stainless Steel Cannula (needle), Silicone oil.
Characteristic:
Different size, meet different requirement
Centric or side nozzle, clear and transparent barrel
Leakage-proof plunger guarantee, special design to prevent the plunger from slipping out
Sterilized by EO gas, non-toxic and pyrogen-free
With or without hypodermic needle
Soft Blister package available.
Other Notes Of 2 Part Syringes
Product ConformanceConform to ISO 7886-1 and ISO 7864In compliance with European Medical Device Directive 93/42/EEC(CE Class: W/needle: lla; W/O needle:l)
Quality AssuranceManufacturing process is in compliance with ISO 13485 and ISO 9001 Quality System.