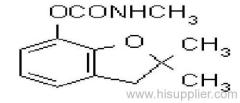
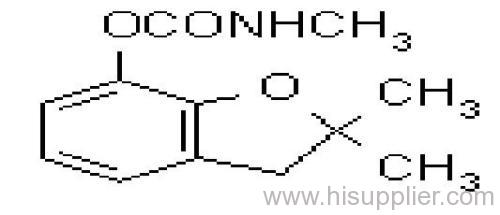
Common name: carbofuran
IUPAC name: 2,3-dihydro-2,2-dimethylbenzofuran-7-yl methylcarbamate
Chemical Abstracts name: 2,3-dihydro-2,2-dimethyl-7-benzofuranyl methylcarbamate
CAS RN: [1563-66-2]; (carbofuran phenol [1563-38-8]; 3-ketocarbofuran phenol [17781-16-7])
EEC no.: 216-353-0
PHYSICAL CHEMISTRY
Mol. wt.: 221.3; M.f.: C12H15NO3; Form: Colourless crystals. M.p.: 153-154 ºC; (tech. 150-152 ºC); V.p.: 0.031 mPa (20 ºC); 0.072 mPa (25 ºC). KOW: logP = 1.52 (20 ºC); S.g./density: 1.18 (20 ºC). Solubility: In water 320 (20 ºC), 351 (25 °C) (both in mg/l). In dichloromethane >200, isopropanol 20-50, toluene 10-20 (all in g/l, 20 ºC). Stability: Unstable in alkaline media. Stable in acidic and neutral media. Decomposes >150 ºC. DT50 (22 ºC) >1 y (pH 4), 121 d (pH 7), 31 h (pH 9).
APPLICATIONS
Biochemistry: Cholinesterase inhibitor.
Mode of action: Systemic, with predominantly contact and stomach action.
Uses: Control of soil-dwelling and foliar-feeding insects (including wireworms, white grubs, millipedes, symphylids, frit flies, bean seed flies, root flies, flea beetles, weevils, sciarid flies, aphids, thrips, etc.) and nematodes in vegetables, ornamentals, beet, maize, sorghum, sunflowers, oilseed rape, potatoes, alfalfa, peanuts, soya beans, sugar cane, rice, cotton, coffee, cucurbits, tobacco, lavender, citrus, vines, strawberries, bananas, mushrooms, and other crops.
Formulation types: FS; GR; SC; WP.
Compatibility: Incompatible with alkaline materials.
MAMMALIAN TOXICOLOGY
Oral: Acute oral LD50 for male and female rats c. 8, dogs 15, mice 14.4 mg/kg.
Skin and eye: Acute percutaneous LD50 (24 h) for male and female rats >2000 mg/kg; mildly irritating to skin and eyes (rabbits).
Inhalation: LC50 (4 h) for male and female rats c. 0.075 mg/l air (aerosol).
NOEL: (2 y) for rats and mice 20 mg/kg diet; (1 y) for dogs 10 mg/kg diet.
ADI: 0.002 mg/kg b.w.
Water GV: 7g/l (based on ADI).
Toxicity class: WHO (a.i.) Ib; EPA (formulation) I ('Furadan 4F'), II ('Furadan G')
EC hazard: T+; R26/28| N; R50, R53
ECOTOXICOLOGY
Birds: Acute oral LD50 for Japanese quail 2.5-5 mg/kg. LC50 for Japanese quail 60-240 mg (as GR5)/kg. Tech.: LC50 0.7-8 mg/kg, depending on species.
Fish: LC50 (96 h) for rainbow trout 22-29 mg (as GR5)/l, bluegill sunfish 1.75 mg (as GR3)/l, golden orfe 107-245 mg (as GR5)/l. Tech.: 7.3-362.5 g/l, depending on species.
Daphnia: LC50 (48 h) 38.6 g/l.
Bees: Toxic to bees (except for granular formulation).
ENVIRONMENTAL FATE
Animals: Carbofuran is metabolised by hydrolytic and oxidative mechanisms in the rat. At 24 hours after treatment, 72% of the dose was eliminated in the urine, 2% in the faeces, and about 43% of the administered dose was hydrolysed. Over 95% of the material excreted in the urine was in the form of conjugated metabolites. The major metabolite was conjugated 3-ketocarbofuran phenol, while conjugated 3-hydroxycarbofuran was the predominant carbamate metabolite. Both metabolites were also present in the free form. Metabolism of carbamate insecticides is reviewed.
Plants: Carbofuran is quickly metabolised into 3-hydroxycarbofuran and ketocarbofuran.
Soil/Environment: DT50 in soil c. 30-60 d. Most important metabolite is CO2 formed by microbiological degradation of the phenol compounds. Koc 22.