Common name: bioallethrin; depalléthrine; d-trans-allethrin
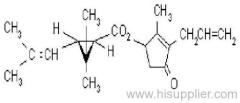
IUPAC name: (RS)-3-allyl-2-methyl-4-oxocyclopent-2-enyl (1R,3R)-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate Alt: (RS)-3-allyl-2-methyl-4-oxocyclopent-2-enyl (+)-trans-chrysanthemate
Roth: (RS)-3-allyl-2-methyl-4-oxocyclopent-2-enyl (1R)-trans-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate
Chemical Abstracts name: 2-methyl-4-oxo-3-(2-propenyl)-2-cyclopenten-1-yl 2,2-dimethyl-3-(2-methyl-1-propenyl)cyclopropanecarboxylate
CAS RN: [584-79-2] (same as for allethrin)
EEC no.: 209-542-4
PHYSICAL CHEMISTRY
Composition: Bioallethrin contains 93% m/m total isomers, of which there is 90% trans- isomer pair and 3% cis- isomer pair. Material in 'D-Trans' is 90% m/m bioallethrin. For d-allethrin, see allethrin [(1R)- isomers]. Mol. wt.: 302.4; M.f.: C19H26O3; Form: Bioallethrin is an orange-yellow viscous liquid. The material in 'D-Trans' is an amber viscous liquid. M.p.: Not applicable; no crystallisation observed at -40 ºC. B.p.: Bioallethrin 165-170 ºC/0.15 mmHg; V.p.: 43.9 mPa (25 ºC); KOW: logP = 4.68 (25 ºC); Henry: 2.89 Pa m3 mol-1 (calc.) S.g./density: 1.012 at 20 ºC; Solubility: Bioallethrin: in water 4.6 mg/l (25 ºC). Completely miscible with acetone, ethanol, chloroform, ethyl acetate, hexane, toluene and dichloromethane (20 ºC). Stability: Degraded by u.v. light. In aqueous solution, DT50 1410.7 d (pH 5), 547.3 d (pH 7), 4.3 d (pH 9). Specific rotation: [a]D20 -18.5 to -22.5 (50 g/l toluene); F.p.: 87 ºC (Pensky-Martens)
APPLICATIONS
Mode of action: Non-systemic, non-residual insecticide with contact and stomach action. Has rapid knockdown activity.
Uses: Bioallethrin is a potent contact, non-systemic, non-residual insecticide which produces a rapid knockdown and is used against household insect pests (Blattodea, Culicidae and Muscidae) and in insecticidal coils and electric thermal vapourisers. Used in mixtures with other insecticides and piperonyl butoxide. The (S)-cyclopentenyl ester is the more potent form.
Formulation types AE; EC; OL; TC; VP; Oil; Coil; Mat.
MAMMALIAN TOXICOLOGY
Oral: Acute oral LD50 for male rats 709 mg bioallethrin/kg, female rats 1042 mg/kg; for male rats 425-575 mg ('D-Trans')/kg, female rats 845-875 mg/kg.
Skin and eye: Acute percutaneous LD50 for rabbits >3000 mg bioallethrin/kg.
Inhalation: LC50 (4 h) for rats 2.5 mg bioallethrin/l air.
NOEL: (90 d) for rats 750 mg/kg diet.
Other: No mutagenic, carcinogenic, embryotoxic or teratogenic effects have been observed.
Toxicity class: WHO (a.i.) II; EPA (formulation) II (tech.), III (formulations)
EC hazard Xn; R20/22| N; R50, R53
ECOTOXICOLOGY
Birds: Acute oral LD50 for bobwhite quail 2030 mg/kg.
Fish: Highly toxic. LC50 (96 h) (static test, flow-through test) for coho salmon 22.2, 9.40, steelhead trout 17.5, 9.70, channel catfish >30.1, 27.0, yellow perch -, 9.90g/l.
Daphnia LC50 (96 h) 0.0356 mg/l.
ENVIRONMENTAL FATE
Animals: [14C-acid]-bioallethrin administered in rats with a single dose of either 200 or 100 mg/kg b.w. was readily eliminated in the urine and faeces of all dose groups within 2-3 days after treatment.
Soil/Environment: [14C-alcohol]-bioallethrin degrades in pH 5 buffer to allethrolene, dihydroxyallethrolene, CO2 and a number of small yield polar products as the result of photochemical processes. Cis/trans isomerisation was not observed. The actual and extrapolated DT50 of light-exposed and dark control samples is 48.8 h and 1447 h, respectively.